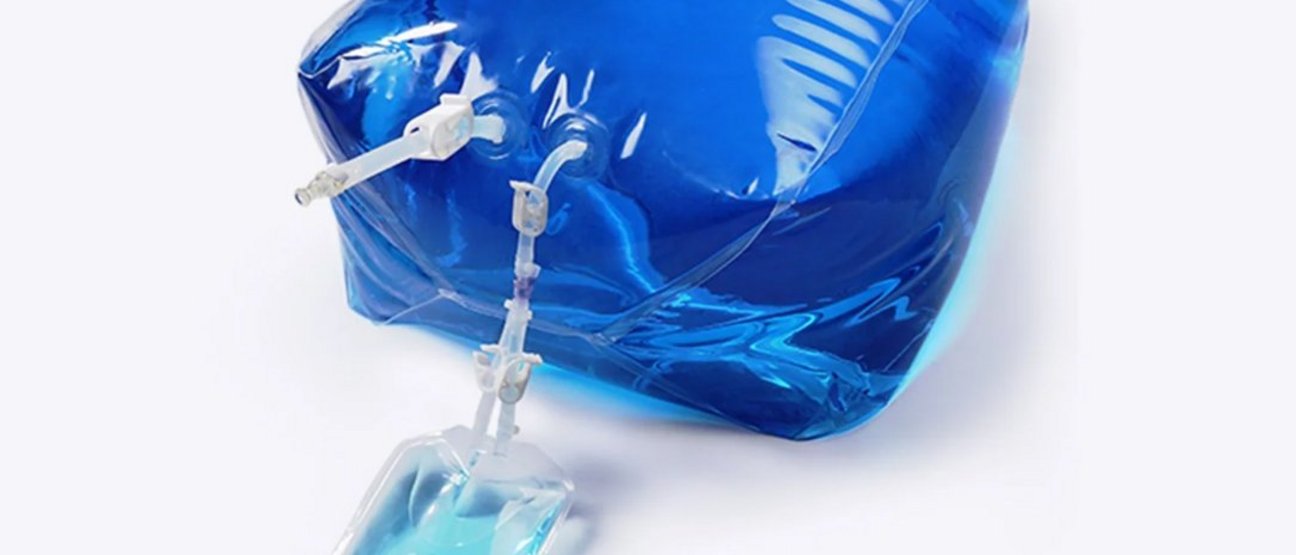
Single-Use Liquid Storage and Shipping Bags

We offer the Lifecube® 2D/3D Standard Single-Use Storage and Shipping Bags in a range of sizes from 50 ml to 3000 l for the biopharmaceutical industry. Our single-use bioprocess bags are constructed of a five-layer Lifemeta® KA film, with ULDPE as the fluid contact material and EVOH as the gas barrier layer. The outer protective HDPE layer is to optimize overall toughness of the bag. These single-use bioprocess bags are produced in ISO Class 7 cleanroom that is ISO 9001 compliant.
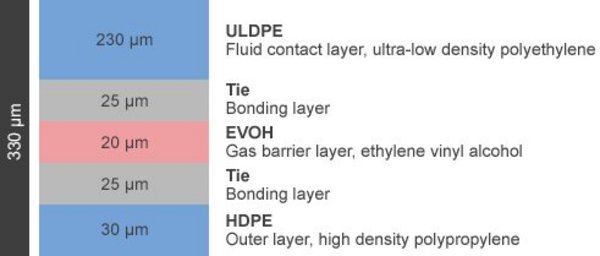
Lifemeta® KA Film (Blown Film)
The Lifemeta® KA films of single-use bags are composed of five layers.
Quality Assurance
- ISO 9001:2015 quality management system
- ISO Class 7 clean zone
- 100% leak testing
- ADCF raw materials
- Meet FDA Indirect Food Additive requirements cited in 21 CFR 177-182
- Meet requirement of USP <87> In Vitro Biological Reactivity Test
- Meet requirement of USP <88> Biological Reactivity Test for Class VI plastics
- Aqueous extraction contains < 0.25 EU/ml as determined by Limulus Amebocyte Lysate (LAL), USP <85>
- Particulate matter in the product eluent meets the requirements in USP <788> for large volume parenterals
- Verify gamma irradiation dose according to ISO 11137
- Provide product validation guide and certificate of quality
Features
- Good physical strength and excellent biological safety
- Integrated bag body designed for Lifecube® 2D/3D single-use bags and less residual liquid
- Double-layer sterile packaging sterilized by 25-45 kGy gamma irradiation
- Variable and flexible customized services
- Various and scalable
Typical Applications
- Buffer and media storage and transfer after filtration
- Intermediates storage
- Stock solution storage
- Formulation filling